Blood Specimen Collection and Processing Phlebotomy
The first step in acquiring a quality lab test result for any patient is the specimen collection procedure. The venipuncture procedure is complex, requiring both knowledge and skill to perform. Several essential steps are required for every successful collection procedure:
Vein puncture Procedure:
- 1A phlebotomist must have a professional, courteous, and understanding manner in all contact with all patients.
- 2The first step to the collection is to positively identify the patient by two forms of identification; ask the patient to state and spell his/her name and give you his/her birth date. Check these against the requisition (paper or electronic).
- 3Check the requisition form for requested tests, other patient information and any special draw requirements. Gather the tubes and supplies that you will need for the draw.
- 4Position the patient in a chair, or sitting or lying on a bed.
- 5 Wash your hands and sanitize.
- 6Select a suitable site for vein puncture, by placing the tourniquet 3 to 4 inches above the selected puncture site on the patient. Do not put the tourniquet on too tightly or leave it on the patient longer than 1 minute.
- 7Next, put on non-latex gloves, and palpate for a vein.
- 8When a vein (median cubital vein or Cephalic vein) is selected, cleanse the area with cotton swab wetted with surgical spirit in a circular motion, beginning at the site and working outward. Allow the area to air dry. After the area is cleansed, it should not be touched or palpated again. If you find it necessary to reevaluate the site by palpation, the area needs to be re-cleansed before the venipuncture is performed.
- 9Ask the patient to make a fist; avoid “pumping the fist.” Grasp the patient’s arm firmly using your thumb to draw the skin taut and anchor the vein. Swiftly insert the needle (use Sterile disposable syringe and 21 or 22 size needle) through the skin into the lumen of the vein. The needle should form a 15-30 degree angle with the arm surface. Avoid excess probing.
- 10When the last tube is filling, remove the tourniquet.
- 11Remove the needle from the patient's arm using a swift backward motion..
- 12Place gauze immediately on the puncture site. Apply and hold adequate pressure to avoid formation of a hematoma. After holding pressure for 1-2 minutes, tape a fresh piece of gauze or Band-Aid to the puncture site
- 13Dispose of contaminated materials/supplies in designated containers.
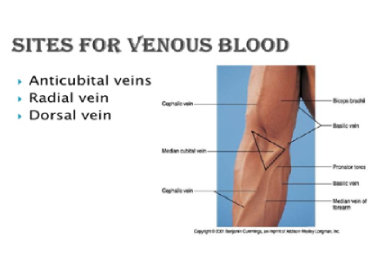
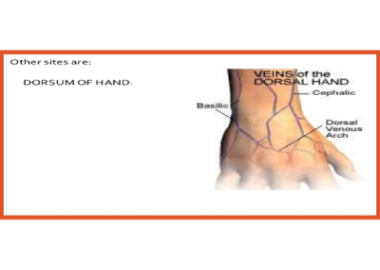
Note: The larger median cubital and cephalic veins are the usual choice, but the basilic vein on the dorsum of the arm or dorsal hand veins are also acceptable. Foot veins are a last resort because of the higher probability of complications. Please see the below pictures of hand showing the vein positions.

Fingerstick Procedure:
- 1Follow the procedure for venipuncture as outlined above.
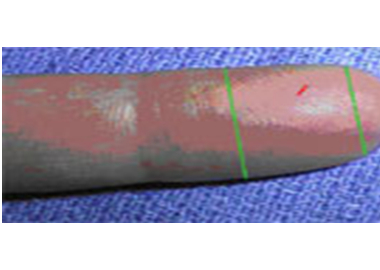
- 2The best locations for fingersticks are the 3rd (middle) and 4th (ring) fingers of the non-dominant hand. Do not use the tip of the finger or the center of the finger. Avoid the side of the finger where there is less soft tissue, where vessels and nerves are located, and where the bone is closer to the surface. The 2nd (index) finger tends to have thicker, callused skin. The fifth finger tends to have less soft tissue overlying the bone. Avoid puncturing a finger that is cold or cyanotic, swollen, scarred, or covered with a rash.
- 3When a site is selected, put on gloves, and cleanse the selected puncture area.
- 4Massage the finger toward the selected site prior to the puncture.
- 5 Using a sterile safety lancet, make a skin puncture just off the center of the finger pad. The puncture should be made perpendicular to the ridges of the fingerprint so that the drop of blood does not run down the ridges.
- 6Wipe away the first drop of blood, which tends to contain excess tissue fluid.
- 7Collect drops of blood into the collection tube/device by gentle pressure on the finger. Avoid excessive pressure or “milking” that may squeeze tissue fluid into the drop of blood.
- 8 Cap, rotate and invert the collection device to mix the blood collected.
- 9Have the patient hold a small gauze pad over the puncture site for a few minutes to stop the bleeding.
- 10Dispose of contaminated materials/supplies in designated containers.
- 11Label all appropriate tubes at the patient bedside.
Heelstick Procedure (infants):
The recommended location for blood collection on a newborn baby or infant is the heel. Prewarming the infant's heel (42° C for 3 to 5 minutes) is important to increase the flow of blood for collection.
- 1Wash your hands, and put gloves on. Clean the site to be punctured with an alcohol sponge. Dry the cleaned area with a dry gauze pad.
- 2 Hold the baby's foot firmly to avoid sudden movement.
- 3Using a sterile blood safety lancet, puncture the side of the heel in the appropriate regions shown above. Make the cut across the heel print lines so that a drop of blood can well up and not run down along the lines.
- 4Wipe away the first drop of blood with a piece of clean, dry cotton gauze. Since newborns do not often bleed immediately, use gentle pressure to produce a rounded drop of blood. Do not use excessive pressure because the blood may become diluted with tissue fluid.
- 5 When finished, elevate the heel, place a piece of clean, dry cotton on the puncture site, and hold it in place until the bleeding has stopped. Apply tape or Band-Aid to area if needed.
- 6Wipe away the first drop of blood, which tends to contain excess tissue fluid.
- 7Be sure to dispose of the lancet in the appropriate sharps container. Dispose of contaminated materials in appropriate waste receptacles.
- 8 Remove your gloves and wash your hands.
Order of Draw Blood collection tubes must be drawn in a specific order to avoid cross-contamination of additives between tubes. The recommended order of draw is:
First - blood culture bottle or tube (yellow or yellow-black top)
Second - coagulation tube (light blue top).
- 1Third - non-additive tube (red top)
- 2 Last draw - additive tubes in this order:
- SST (red-gray or gold top). Contains a gel separator and clot activator.
- Sodium heparin (dark green top)
- PST (dark green top with gold rim). Contains lithium heparin anticoagulant and a gel separator.
- EDTA (lavender top)
- Oxalate/fluoride (light gray top) or other additives
- NOTE: Tubes with additives must be thoroughly mixed. Clotting or erroneous test results may be obtained when the blood is not thoroughly mixed with the additive.
| Tube cap color | Additive | Function of Additive | Common laboratory tests |
|---|---|---|---|
Light-blue |
3.2% Sodium citrate | Prevents blood from clotting by binding calcium | Light Blue Cap- Coagulation Factors Tests, D- dimer, PT, PTT |
Red or gold (mottled or "tiger" top used with some tubes is not shown) |
Serum tube with or without clot activator or gel | Clot activator promotes blood clotting with glass or silica particles. Gel separates serum from cells. | Gold Cap-Clot activator-Bio Chemistry panel Tests, serology, immunology Tests. Red Top cap(Plain)-Toxicology, Drug Monitoring, Electrolytes, Lipid Profile, RFT, Cardiac Profile, Enzymes, Proteins, Bio Markers, Cancer Markers, TFT, Metabolic Disorders, Serology and Immunological Tests. Etc |
Green |
Sodium or lithium heparin with or without gel | Prevents clotting by inhibiting thrombin and thromboplastin | Green cap Lithium Heparin- Stat and Routine Bio chemistry,Ammonia & carboxyhemoglobin, etc Green capSodium Heparin_HBA1c, Homocyatein, HIV-viral load & T-cell helper /suppressor,DAT and AntibodyTiter |
Lavender or pink |
Potassium EDTA | Prevents clotting by binding calcium | Lavender Cap-Hematology,CBC, Blood smear preparation, Heamogram Pink capBlood bank cross match |
Gray |
Sodium fluoride, and sodium or potassium oxalate | Fluoride inhibits glycolysis, and oxalate prevents clotting by precipitating calcium. | Gray Cap - FBS/PPBS/RBS, OGCT, Glucose (especially when testing will be delayed), blood alcohol, lactic acid |
Labeling The Sample
All specimens must be received along with Lab request form by the laboratory with a legible label containing 1) Patient Name
2) Clinic / Hospital ID
3) Lab ID and
4)Tests Name
Techniques to Prevent Hemolysis (which can interfere with many tests):
- Mix all tubes with anticoagulant additives gently (vigorous shaking can cause hemolysis) 5-10 times.
- Avoid drawing blood from a hematoma; select another draw site.
- If using a needle and syringe, avoid drawing the plunger back too forcefully.
- Make sure the venipuncture site is dry before proceeding with draw.
- Avoid a probing, traumatic venipuncture.
- Avoid prolonged tourniquet application (no more than 2 minutes; less than 1 minute is optimal).
- Avoid massaging, squeezing, or probing a site
- Avoid excessive fist clenching.
- If blood flow into tube slows, adjust needle position to remain in the center of the lumen.
Blood Sample Handling and Processing:
Pre-centrifugation Handling - The first critical step in the lab testing process, after obtaining the sample, is the preparation of the blood samples. Specimen integrity can be maintained by following some basic handling processes:
- Fill tubes to the stated draw volume to ensure the proper blood-to-additive ratio. Allow the tubes to fill until the vacuum is exhausted and blood flow ceases.
- Tubes should be stored at 4-25°C (39-77°F).
- Tubes should not be used beyond the designated expiration date.
- Mix all gel barrier and additive tubes by gentle inversion 5 to10 times immediately after the draw. This assists in the clotting process. This also assures homogenous mixing of the additives with the blood in all types of additive tubes.
- Serum separator tubes should clot for a full 30 minutes in a vertical position prior to centrifugation. Short clotting times can result in fibrin formation, which may interfere with complete gel barrier formation.
Blood Sample Centrifugation - It is recommended that serum be physically separated from contact with cells as soon as possible, with a maximum time limit of 2 hours from the time of collection.Complete gel barrier formation (gel barrier tubes) is time, temperature and G-force
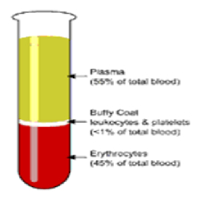
- In general, for a horizontal, swing-bucket centrifuge, the recommended spin time is 10 minutes. For a fixed-angle centrifuge, the recommended spin time is 15 minutes.
- NOTE: Gel flow may be impeded if chilled before or after centrifugation.
- Tubes should remain closed at all times during the centrifugation process.
- Place the closed tubes in the centrifuge as a "balanced load" noting the following:
* Opposing tube holders must be identical and contain the same cushion or none at all.
* Opposing tube holders must be empty or loaded with equally weighted samples (tubes of the same size and equal in fill).
* If an odd number of samples is to be spun, fill a tube with water to match the weight of the unpaired sample and place it across from this sample.
Centrifuge Safety
- Interference with an activated centrifuge by an impatient employee can result in bodily injury in the form of direct trauma or aerosolization of hazardous droplets.
- Centrifuges must never be operated without a cover in place.
- Uncovered specimen tubes must not be centrifuged.
- Centrifuges must never be slowed down or stopped by grasping part(s) of the device with your hand or by applying another object against the rotating equipment.
- Be sure the centrifuge is appropriately balanced before activating. If an abnormal noise, vibration, or sound is noted while the centrifuge is in operation, immediately stop the unit (turn off the switch) and check for a possible load imbalance.
- Clean the centrifuge daily with a disinfectant and paper towel. Broken tubes or liquid spills cleaned with proper precautions.
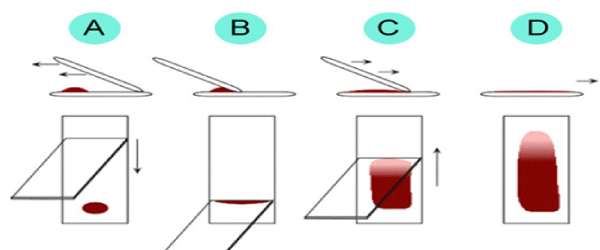
BLOOD SMEAR PREPARATION ON GLASS SLIDE FOR BLOOD CELL STUDY: Blood Smear Preparation Guidelines Place a drop of capillary or well-mixed EDTA blood in the center of one end of a clean and grease free glass slide. Immediately place a spreader slide just in front of the drop of blood. Draw the spreader slide back into the drop of blood, lower to about a 30° angle, and allow drop to flow evenly across the spreader slide edge. Push the spreader slide quickly over the length of the specimen slide in one even motion. Dry immediately and completely with gentle fanning or waving. Label with two patient identifiers (for example, clinic / Lab / Patient ID) using a lead pencil. Do not place slides in refrigerator or freezer.